
2020-02-26
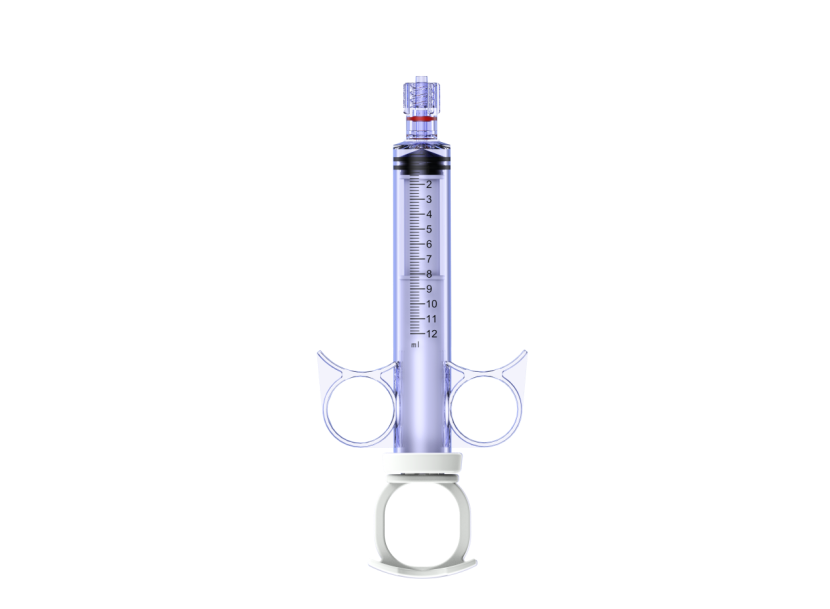
Indications for Use Statement
Angiography Syringe is used to inject contrast media into the heart, great vessels, and coronary arteries during angiographic procedures.
About Angiography Syringe
510(k) Number: K170027
Regulation Number: 870.1650
Classification Product Code: DXT
Date Received: 01/03/2017
Decision Date: 10/03/2017
Decision: Substantially Equivalent (SESE)
Regulation Medical Specialty: Cardiovascular
510k Review Panel: Cardiovascular
About INT Medical
Shanghai Kindly Medical Instruments Co., Ltd. was established in 2006. Kindly owns more than 600 employees. The headquarter is located in Jiading, Shanghai, manufacturing cardiovascular, peripheral and neuro intervention products approved by FDA (510K), with a complete industrial chain of independent mold processing, product research and development, equipment development, sterilization, etc..